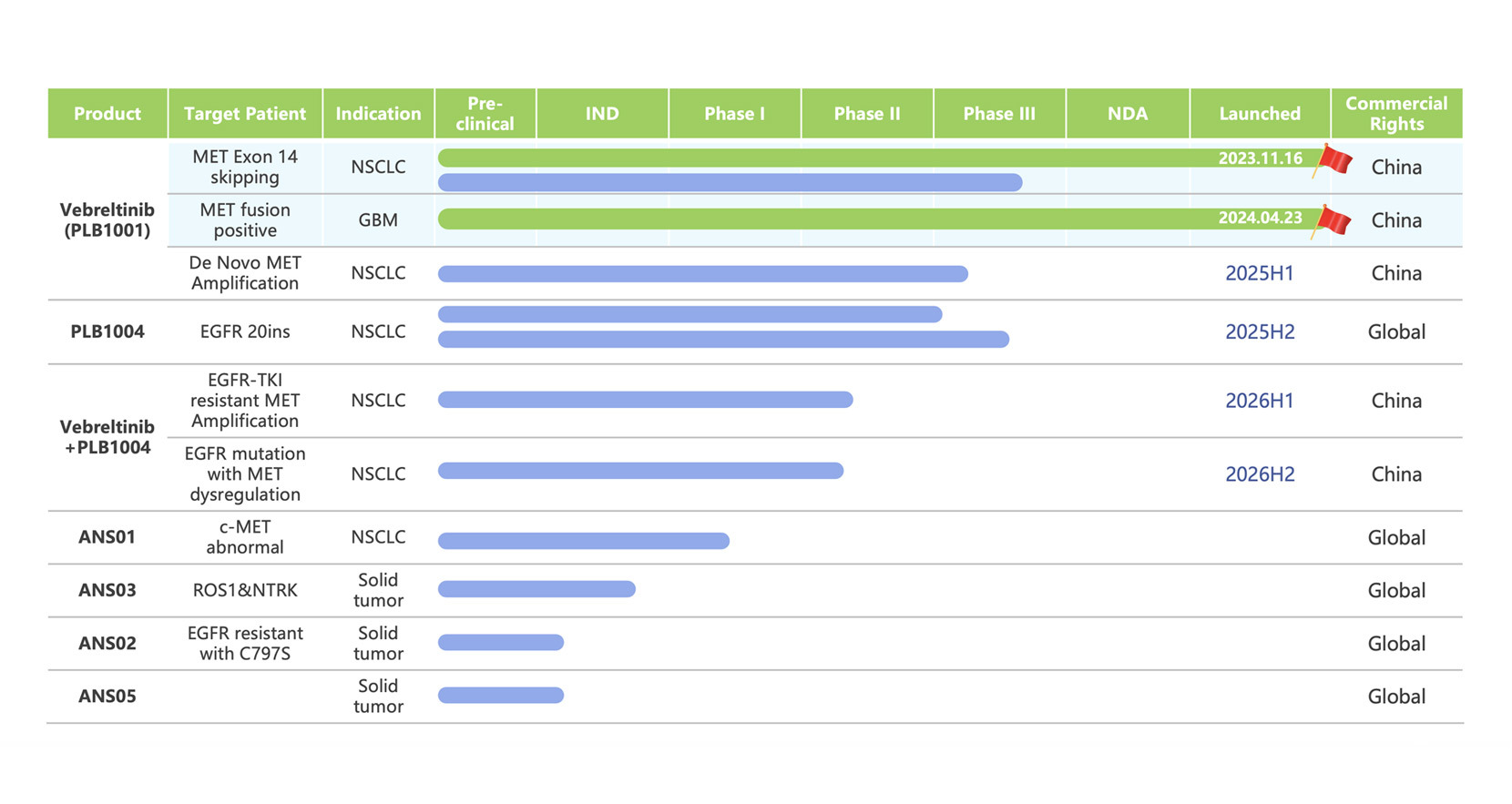
Product pipeline

Recruit

分类筛选
条件:
清空条件
Currently Enrolling
Phase I multiple-center, open-label, dose escalation and dose expansion study for assessing the safety, tolerability, pharmacokinetics and anti-tumor effect of PLB1004 in treatment of advanced non-small cell lung cancer
Indication:
Cancer species:non-small cell lung cancer
Time:2022-09-26
Currently Enrolling
A multi-center, multi-queue, open Phase II clinical trial to evaluate the effectiveness and safety of Vebreltinib enteric capsules on late-stage non-small cell lung cancer with c-Met irregularity.
Indication:Treatment of adults with locally advanced or metastatic non-small cell lung cancer with interstitial epithelial transforming factor (MET) exon 14 jump
1. Execution of letter of informed consent;
2. Male or female of age 18 and above;
Cancer species:non-small cell lung cancer
Time:2022-09-26
Business development